This site provides INTERCEPT product information for International audiences Select your region
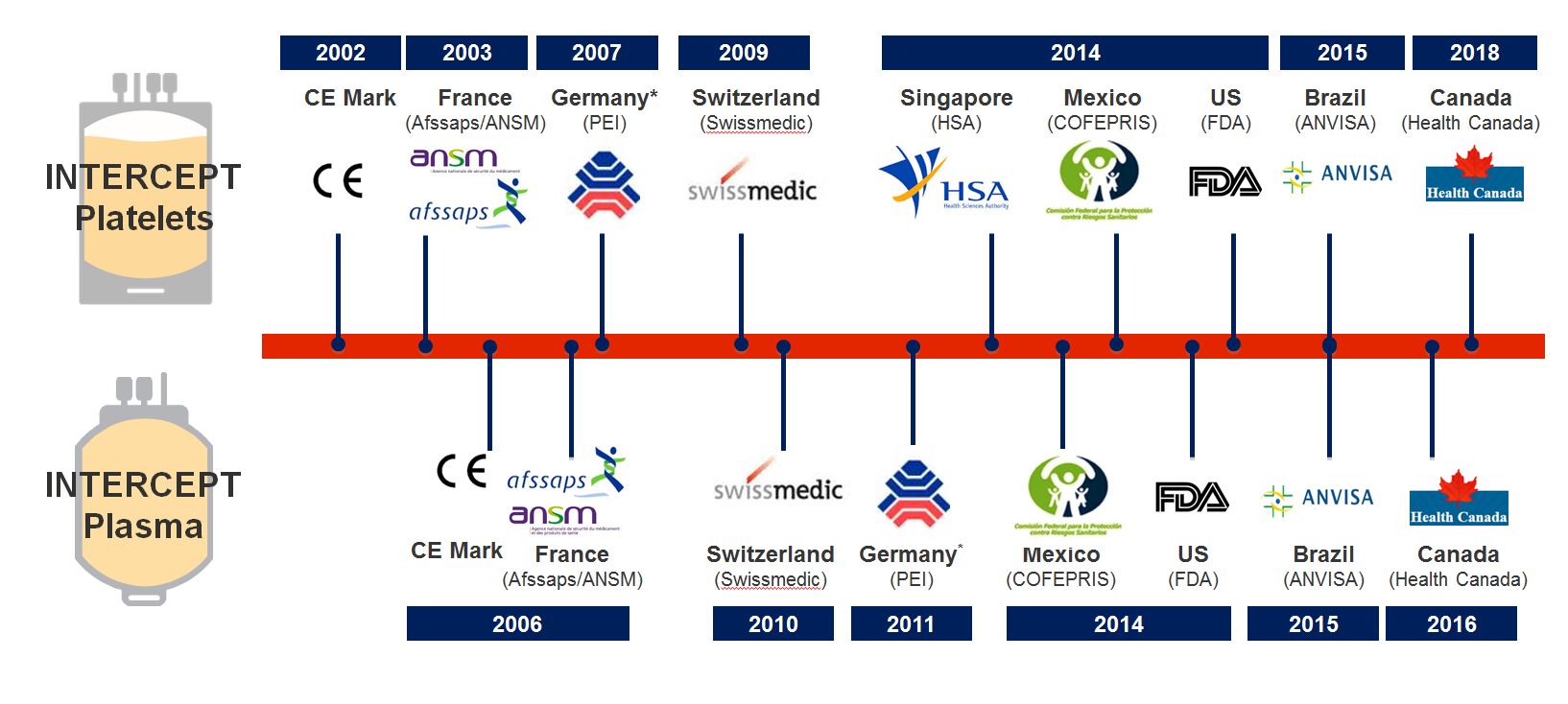
The INTERCEPT™ Blood System for platelets received CE Mark approval in 2002 after demonstrating the clinical safety, efficacy and performance of the device France (ANSM) and Germany approved the use of pathogen inactivated blood components produced with the INTERCEPT Blood System and Switzerland approved the INTERCEPT Blood System technology.
Blood components treated with the INTERCEPT Blood System requires additional approval in France, Germany, and Switzerland. The INTERCEPT Blood System for platelets is the only approved pathogen reduction system in the US. INTERCEPT is currently the only pathogen inactivation method for platelets that has received approval from German, French, Swiss and US authorities for routine use.
Have a look at the INTERCEPT regulatory approval as of October 2019.
When transfusing INTERCEPT-treated platelet concentrates, you know that safety and efficacy have been validated by multiple leading regulatory agencies across the globe.