This site provides INTERCEPT product information for International audiences Select your region
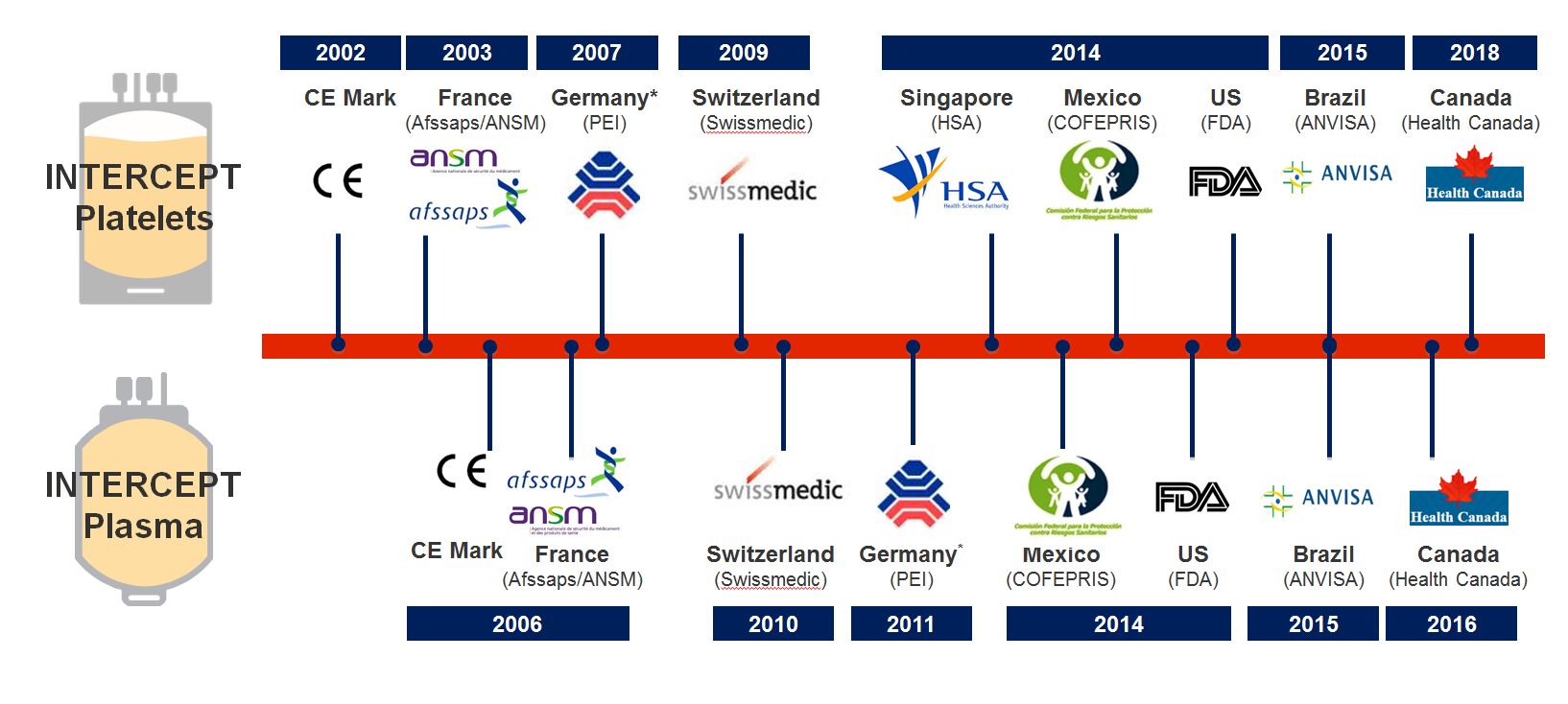
Market Approval by the Most Stringent Regulatory Bodies
The INTERCEPT™ Blood System for platelets received CE Mark approval in 2002. Furthermore INTERCEPT is currently the only pathogen inactivation method for platelets that has received approval from German, French, Swiss and US authorities for routine use.
Have a look at the INTERCEPT regulatory approvals as of October 2019.
When transfusing INTERCEPT-treated platelet concentrates, you know that safety and efficacy have been validated by multiple leading regulatory agencies across the globe.