PATHOGEN INACTIVATION
Safeguard Clinical Outcomes
Blood collected from donors is the only supply available to patients in need
Transfusion needs
Multiple clinical uses increase demand
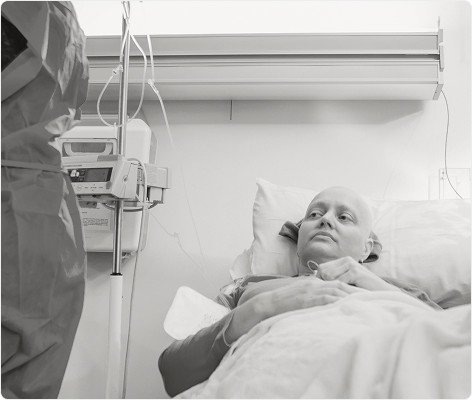
Many patients are transfused with platelet components (PCs) and plasma due to acute needs such as severe trauma from accidents, as well as cancer, organ and bone marrow transplants, cardiovascular and orthopaedic surgeries and inherited blood disorders.

Transfusions Redefined
Protecting vulnerable patients
Components can be given prophylactically or therapeutically, but always with the intention of achieving the best clinical outcome in patients. Multi-transfused patients (MTP) and immuno-compromised patients (ICP) are at a particularly increased risk of transfusion transmitted infections (TTIs) and developing other unwanted complications of blood transfusions.
Avoidable Consequences
The cost of untimely blood availability
The unavailability of timely, safe blood transfusions generates consequences and incurred costs which could be avoided. Examples are:
- Death
- Cancer patients not receiving required transfusions before, during, and after chemotherapy and/or surgery
- Stem cell or organ transplant procedure cancellation or delay
- Surgery cancellation or delay
- Critically injured trauma patients not receiving necessary transfusions on time
Elevated safety and efficacy
INTERCEPT™ Blood System proven in routine use
By reducing the risks of TTIs and certain undesirable adverse events, the INTERCEPT™ Blood System contributes to timely and safe blood transfusions while providing therapeutically effective products. INTERCEPT™ Platelets and Plasma may be prescribed and transfused in accordance with standard transfusion methods.1
You may also be interested in
References:
- The INTERCEPT™ Blood System is European Union Medical Device Regulation (2017/745) approved and is the only U.S. FDA approved pathogen reduction system for platelets and plasma. INTERCEPT™ Blood System received approval by many other regulatory agencies in the world including PEI (Germany), ANSM (France), SwissMedic (Switserland), and HealthCananda (Canada).
There is no pathogen inactivation process that has been shown to eliminate all pathogens. Certain non-enveloped viruses (e.g., HAV, HEV, B19, and poliovirus) and Bacillus cereus spores have demonstrated resistance to the INTERCEPT™ process. For a full list of warnings, precautions for use and pathogens inactivated, please refer to the Technical Data Sheet and Instructions for Use found in the resource section of this website.




