This site provides INTERCEPT product information for International audiences Select your region
How can you take the fast track to improving blood safety?
In Europe, 40% of all platelet concentrates undergo either bacterial screening or pathogen inactivation. This percentage may increase even more in 2018 as major European countries are currently taking action to mitigate the risk of septic transfusion reactions and emerging pathogens.
Perhaps you’d like to follow their example, but you’re not sure how to integrate pathogen inactivation into your existing platelet production processes, or you believe that it’s financially out of reach.
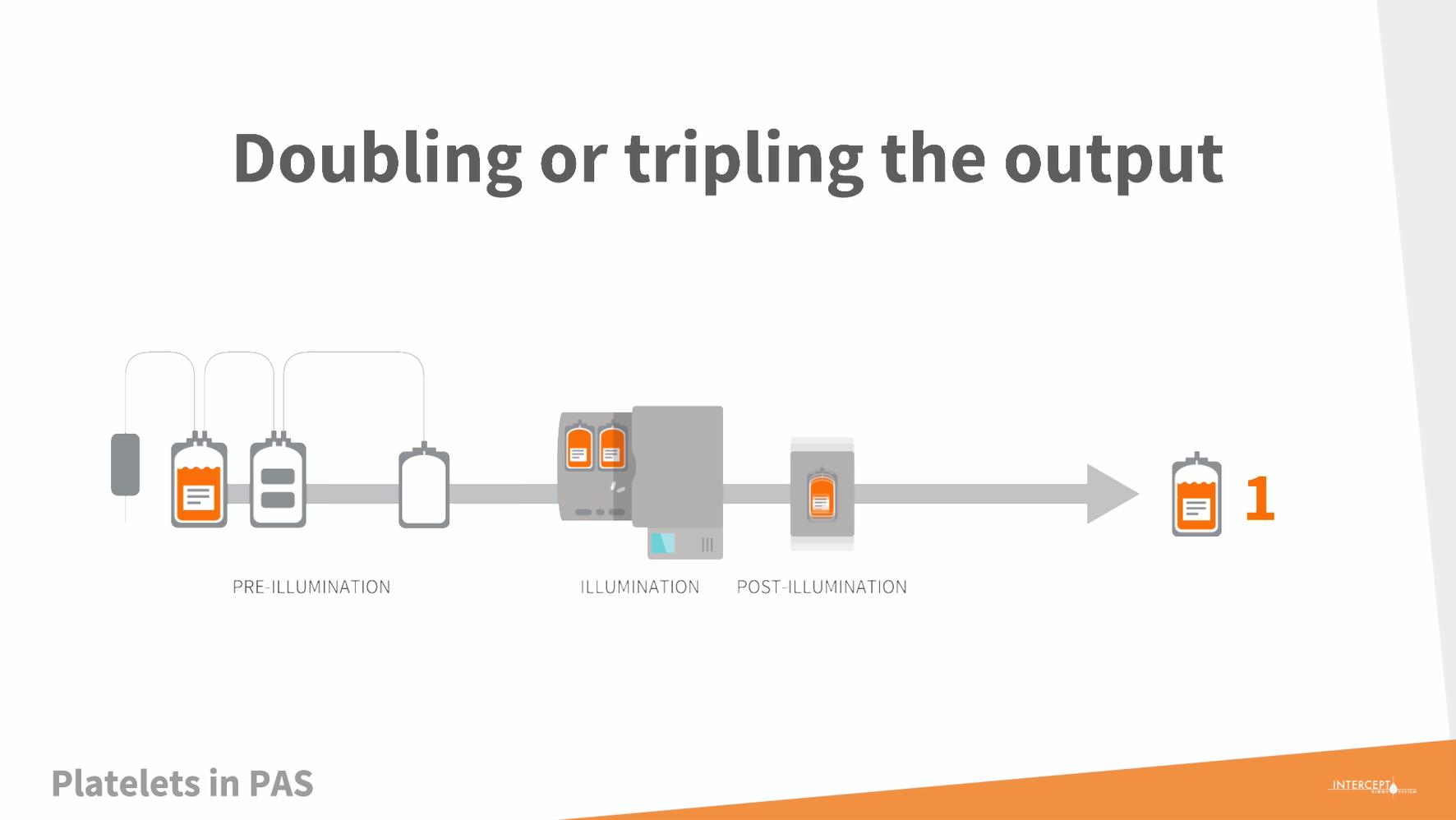
We have good news for you. The INTERCEPT™ Triple Storage Processing Set offers a fast track to improved blood safety.
Would you like to learn more about current blood safety challenges, the benefits of INTERCEPT and the Triple Storage Processing Set?
Click here to view the video.

