This site provides INTERCEPT product information for International audiences Select your region
Double Dose Buffy Coat Platelets: Increasing the Affordability of INTERCEPT™ Pathogen Inactivation
For blood centres seeking a cost-effective way to implement INTERCEPT pathogen inactivation, double dose buffy coat platelet production may offer a solution.
What is a double dose buffy coat platelet unit?
A platelet concentrate with sufficient platelet count to result in 2 therapeutic doses after pathogen inactivation with the INTERCEPT Blood System.
The double dose buffy coat platelet production process can contribute significantly to the affordability of the INTERCEPT Blood System for platelets, offering direct, as well as indirect financial and economic advantages.
Cost Reductions Due to Double Dose
Buffy Coat Platelet Production
There are several ways in which the pooling of 7 to 8 optimised buffy coats may contribute to the affordability of the INTERCEPT Blood System for platelets. In addition to a potential lower number of buffy coats required to obtain 2 therapeutic doses, the required number of platelet pooling sets, platelet additive solution (PAS) and INTERCEPT processing sets can be reduced by 50%, and the number of sterile docks required can be reduced by up to 30%.
On top of these savings, the double dose buffy coat platelet production process can add to logistical and economic advantages: treating 2 therapeutic doses at once reduces the number of illuminations by 50%, equally saving on hands-on time. This, in turn, can translate into economic value.
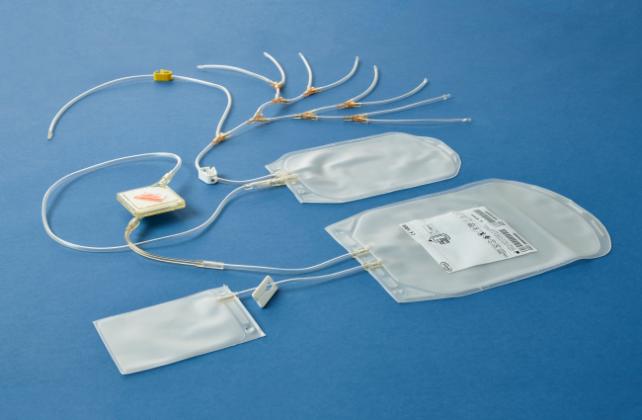
In addition, Cerus offers the know-how as well as specially designed disposables to support the transition from single to double dose buffy coat platelet production. Learn more about the total approach offered by Cerus and the specially designed I-Platelet Pooling Set.
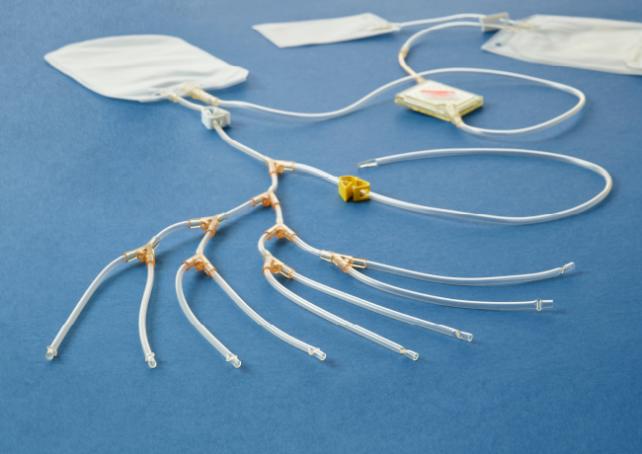
Cerus Offers Tailored Tools
and Comprehensive Support
The I-Platelet Pooling Set is specially designed for double dose production of leuko-reduced platelets from whole-blood-derived buffy coat in additive solution and is validated to pool 7 to 8 buffy coats.
The total approach offered by Cerus relies on the skilled, well-trained and experienced Cerus Deployment team. They have developed a sound methodology to support blood centres in the implementation of the INTERCEPT Blood System for platelets in a cost-effective way. The team members have extensive experience with successful implementations in blood centres of all sizes, transitioning from semi-automated as well as manual production methods. Based on this experience, they thoroughly assess the feasibility of the double dose buffy coat platelet approach in every blood centre, and assist with the implementation.
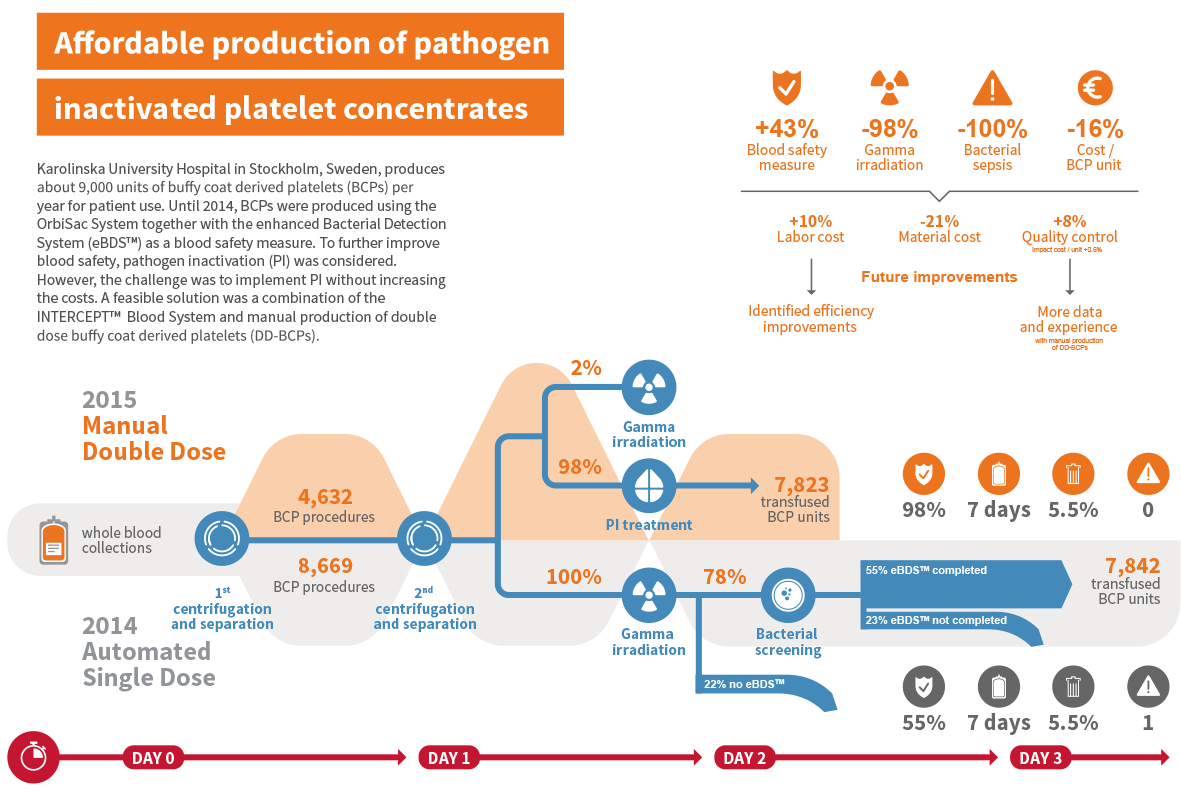
The Karolinska University Hospital in Stockholm (Sweden) is an excellent example of a blood centre that managed to implement pathogen inactivation in a cost-neutral, and long term cost-savings by switching from semi-automated single dose platelet production to manual double dose buffy coat platelet production.
Would you like more information?
Click here to receive information or contact a Cerus representative.
Please send me additional information on:
Contact me to discuss the possibilities of implementing the INTERCEPT Blood System for platelets in a cost-effective way in my blood centre.
The INTERCEPT Blood System is not approved for sale in certain countries.